Suitestensa RIS
Radiology information system for hospital practice
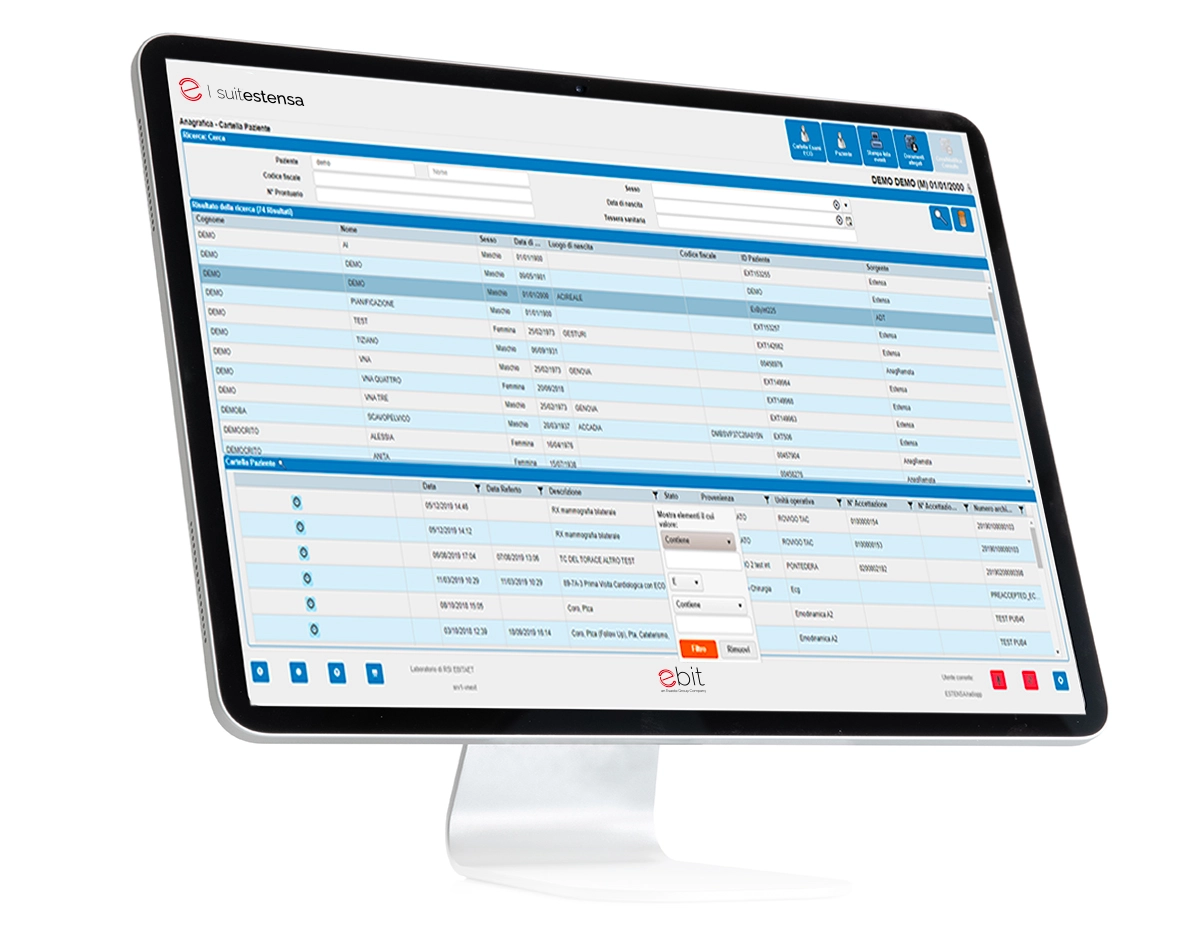
SUITESTENSA RIS is the all-in-one solution for the management of all data, examinations and images generated as part of the hospital practice: the phases of booking, analysis, reporting and distribution of data to non-radiological departments and end users are all part of the SUITESTENSA workflow.
Main features
Perfect integration with hospital information systems
Natively designed according to HL7 and DICOM standards
Interactive structured reporting and structured representation of all clinical history data
Customizable and configurable workspaces, interfaces, parameters and layouts

Supports system interoperability
Based on the latest Web technologies, SUITESTENSA RIS uses DICOM 3.0, HL7 and FDA-XML communication protocols to support system interoperability and avoid data duplication. SUITESTENSA RIS PACS implements the latest features on Structured Reporting, 3D and 4D post processing for CT/MRI/PET, including post processing data in the structured report.
SPECIFICATIONS
All features
User interface color adaptable to ambient lighting
Clinical record containing previous examinations, always available during the reporting phase
Scanning module for paper documents, for complete digital archiving of the study
Printing of legal documents and related digitization (National Health System certifications, hand-signed information sheets, etc.)
Patient record and administrative management of hospital admission
Insertion of alarms when a condition occurs (e.g. heredity, surgical procedures, delays in reporting urgent examinations, divergent readings)
Inventory management
Follow-up module with automatic generation of patient recall lists and lists forwarding to modes
Remote and local digital signature
Voice reporting
Electronic reading of patients' health cards
Barcode reading
CD/DVD production, in compliance with the DICOM standard and the IHE-PDI integration profile
Structured database for cross- and multi-parametric researches: information can be preset and exported to customized layouts (integration with Microsoft Excel®)
Customizable Case Report Forms (CRF) for clinical studies: cases can be indexed, anonymized and exported to logical archives
Collected data are compatible with datasets of several national and international scientific associations.
Customer service
REMOTE AND ON-SITE TECHNICAL ASSISTANCE
We provide immediate phone assistance with rapid on-site support as needed to solve your situation.
REAL-TIME MONITORING
We deliver software upgrades to maintain your investment at the current standards.
TRAINING AND EDUCATION
Our Specialists will train your staff to operate the software effectively.
Product details
MANUALS & IFU
The Manual and Instructions for Use (IFU) are included with the device delivery and can be further requested and made available, in paper format, by sending a request to [email protected].
Technology and features are system/configuration dependent. Specifications subject to change without notice. Information might refer to products or modalities not yet approved in all countries. Product images are for illustrative purposes only.
For further details, please contact your Esaote sales representative.
