Suitestensa Endo
Endoscopy Service workflow management
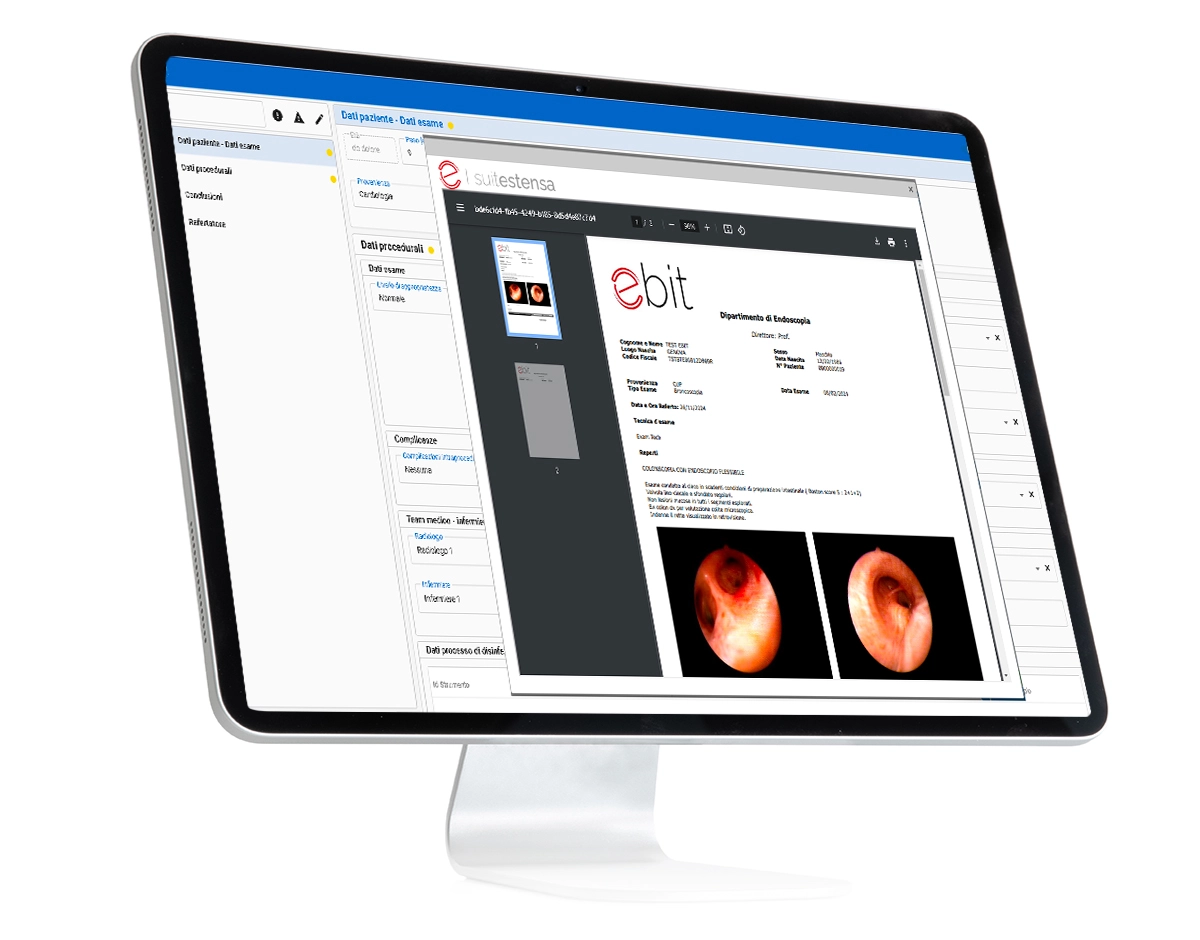
SUITESTENSA Endo is a software dedicated to the management of the workflow within the Endoscopy Service, from acceptance to the reporting of the endoscopic investigation. The SUITESTENSA Endo system's reporting module facilitates the Physician's work from the collection of medical history data to the management of post-examination forms and patient monitoring. If combined with SUITESTENSA Review Diagnostic, the system is able to retrieve in integrated and contextual mode the images and videos previously acquired and archived on the PACS.
Main features
Flexibility: ability to manage each phase of the flow
Configurability: wide configurability possibilities to adapt to the needs of the Facility
Structured Report: associating images, measurements and post-processing
Comparability: the information is presented standardized in a clear and organized format
SPECIFICATIONS
All features
Standardization: clinical data are easily extractable and usable for management, teaching, research and consultation purposes
Collection of medical history data
Collection of examination data: Procedural Data / Instrumentation / Diagnosis Coding
Management of forms: Post-examination and patient monitoring
Collection of configurable and customizable structured information
Report writing Standard phrases, Standard abnormalities, Pre-coded reports, Key images
Patient CD production
Customer service
REMOTE AND ON-SITE TECHNICAL ASSISTANCE
We provide immediate phone assistance with rapid on-site support as needed to solve your situation.
REAL-TIME MONITORING
We deliver software upgrades to maintain your investment at the current standards.
TRAINING AND EDUCATION
Our Specialists will train your staff to operate the software effectively.
Product details
MANUALS & IFU
The Manual and Instructions for Use (IFU) are included with the device delivery and can be further requested and made available, in paper format, by sending a request to [email protected].
Technology and features are system/configuration dependent. Specifications subject to change without notice. Information might refer to products or modalities not yet approved in all countries. Product images are for illustrative purposes only.
For further details, please contact your Esaote sales representative.
