Suitestensa Remote Cardiac Device Management
Implantable devices management software
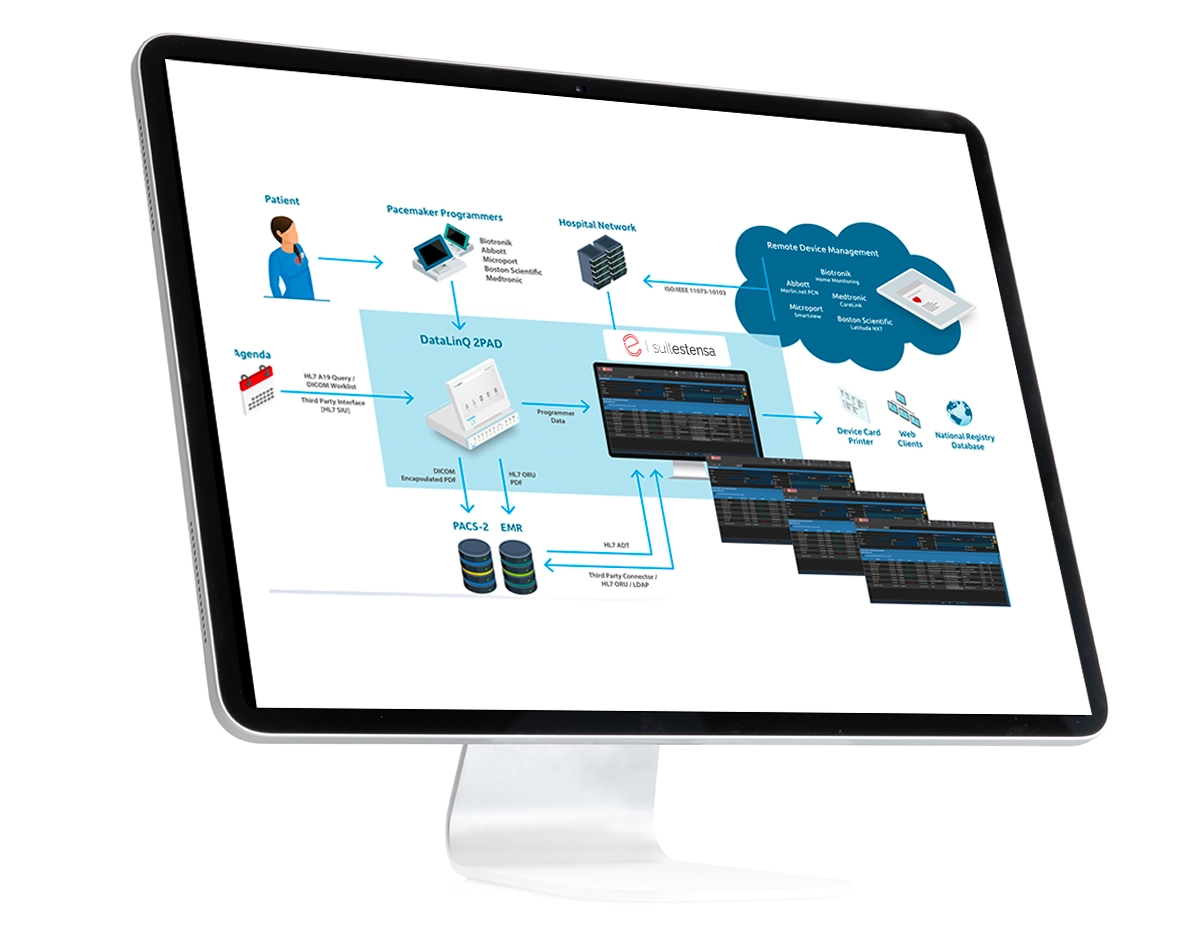
Suitestensa Remote Cardiac Device Management, integrated with the Fysicon DataLinQTM remote control receiver system, offers the solution to properly organize the management of implantable devices such as pacemakers, ICDs, loop recorders monitored remotely, etc. The wide variety of data originated by the different programmers and remotely controlled devices is integrated within the SUITESTENSA database, which allows the management, integrated with hospital information flows, reporting and archiving of data from implantable devices.
Main features
Reception of transmissions from home of remote controls
Dedicated single work list
Import of plant parameters
Archiving on SUITESTENSA database
SPECIFICATIONS
All features
Reception of transmissions from home of remote controls from the main manufacturers of implantable devices
Dedicated single work list for all manufacturers with evidence of alarm codes, thus avoiding having to consult all the portals of the various manufacturers individually to retrieve monitoring information and consult any alarms
Creation of the visit associated with the remote control, associating it with the patient certified registry
Import of plant parameters (generator data, lead), programming/telemetry data, diagnostic data
Matching with reservation received from CUP, for the remote control performance, in order to simplify the reporting operations
Customer service
REMOTE AND ON-SITE TECHNICAL ASSISTANCE
We provide immediate phone assistance with rapid on-site support as needed to solve your situation.
REAL-TIME MONITORING
We deliver software upgrades to maintain your investment at the current standards.
TRAINING AND EDUCATION
Our Specialists will train your staff to operate the software effectively.
Product details
MANUALS & IFU
The Manual and Instructions for Use (IFU) are included with the device delivery and can be further requested and made available, in paper format, by sending a request to [email protected].
Technology and features are system/configuration dependent. Specifications subject to change without notice. Information might refer to products or modalities not yet approved in all countries. Product images are for illustrative purposes only.
For further details, please contact your Esaote sales representative.
