Suitestensa Vascular US
Vascular ultrasound software
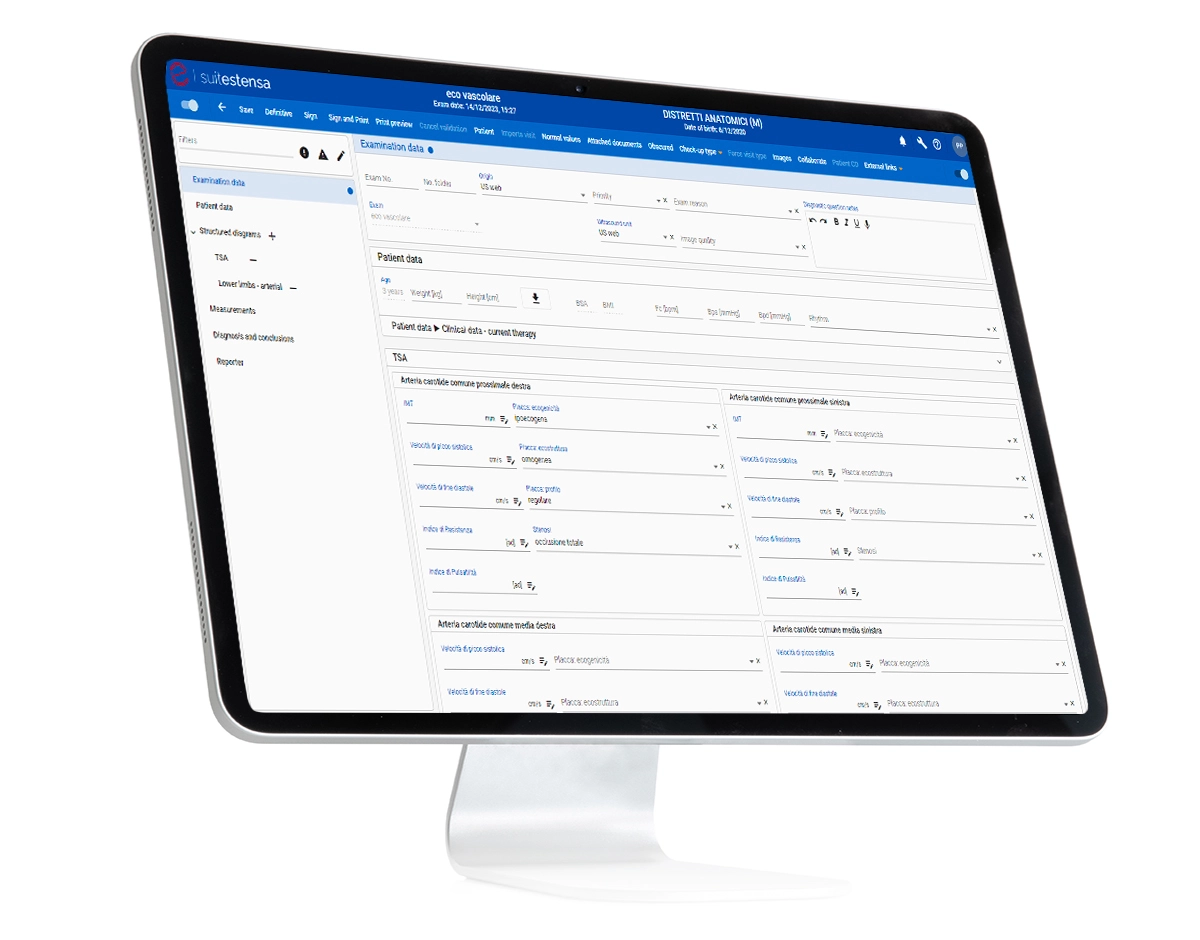
SUITESTENSA Vascular US Data Management is a software dedicated to vascular ultrasound, which allows the management of the workflow of an ultrasound performance path as a whole: from the reception and visualization of images and data, to the drafting and printing of the final report.
Main features
SUPPORT TO ANSWER COMPILATION
PREDEFINED USER TEMPLATES OR FREE TEXT EDITING
FLEXIBLE ORGANIZATION
ORGANIZATION OF ANATOMICAL STRUCTURES
SPECIFICATIONS
All features
Management and automatic import of measurements from US studies in DICOM SR format
Flexible organization (guaranteeing the concept of structured report) which is also easily used by the end user, including all information necessary to ensure the compilation of an examination according to quality standards
ORGANIZATION OF ANATOMICAL STRUCTURES DIVIDED INTO:
Quantitative analyses (B-Mode, M-Mode, Doppler)
Qualitative analyses (structured Morphological descriptions)
Creation and configuration of dedicated referral tabs according to the pathology
Customer service
REMOTE AND ON-SITE TECHNICAL ASSISTANCE
We provide immediate phone assistance with rapid on-site support as needed to solve your situation.
REAL-TIME MONITORING
We deliver software upgrades to maintain your investment at the current standards.
TRAINING AND EDUCATION
Our Specialists will train your staff to operate the software effectively.
Product details
MANUALS & IFU
The Manual and Instructions for Use (IFU) are included with the device delivery and can be further requested and made available, in paper format, by sending a request to [email protected].
