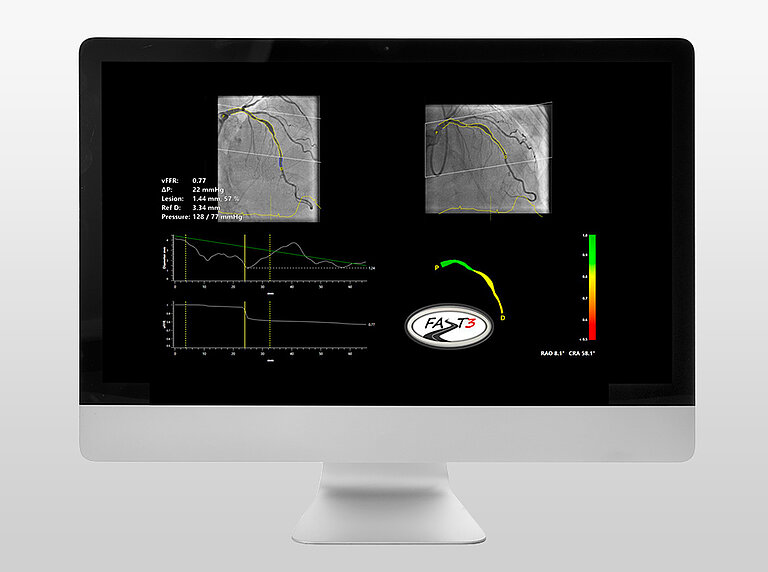
FASTIII is the largest non-inferiority trial running (having enrolled 2228 patients), in which an angiographically derived vFFR guided strategy is compared to a FFR guided strategy to guide coronary revascularization. The primary endpoint is a composite of all-cause death, any myocardial infarction, or any revascularization at 1-year post randomization.
Following the tremendous efforts of the principal investigator, Dr Joost Daemen (cardiologist at Thoraxcenter at the Erasmus University Medical Center, Rotterdam, The Netherlands), over 35 participating centers and ECRI (sponsor of the trial), the patient enrollment has concluded.
“The FASTIII trial is aimed to establish the role of vFFR to coronary revascularization in patients with intermediate coronary artery lesions. An important milestone was reached Friday, May 31st, 2024, when the last patient was enrolled”, said Joost Daemen Principal Investigator of the trial. “The next phase will consist of close follow-up of all patients who generously agreed to participate in the important trial. We hope to present our findings by the end of 2025”.
Additionally, “PMI is committed to providing clinicians and patients with long-term coronary data to inform their treatment decisions,“ said René Guillaume, Managing Director at Pie Medical Imaging. “Our prior studies have shown diagnostic accuracy and reproducibility of vFFR calculation”. FASTIII will establish its role in routine clinical practice.
The trial is funded by research grants from Pie Medical Imaging (Maastricht, The Netherlands) and Siemens Healthineers (Erlangen, Germany). The study is sponsored by ECRI (European Cardiovascular Research Institute, Rotterdam-the Netherlands). Cardialysis (Rotterdam, The Netherlands) is responsible for trial services including trial management and Core Laboratory activities.